Answer: a.
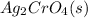
Step-by-step explanation:
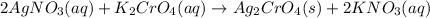
When silver nitrate
 combines with potassium chromate
combines with potassium chromate
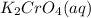 ,they undergo double displacement reaction in which exchange of ions take place.
,they undergo double displacement reaction in which exchange of ions take place.
The products formed are silver chromate
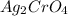 which is insoluble in water and thus formed as precipitate and potassium nitrate
which is insoluble in water and thus formed as precipitate and potassium nitrate
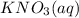 is soluble in water.
is soluble in water.