Answer : The pressure of gas will be, 2904.72 torr
Solution : Given,
First we have to calculate the pressure of oxygen gas and water vapor by using ideal gas equation.

where,
 = pressure of oxygen gas
= pressure of oxygen gas
n = number of moles of gas
 = mass of oxygen gas = 48 g
= mass of oxygen gas = 48 g
 = molar mass of oxygen = 32 g/mole
= molar mass of oxygen = 32 g/mole
V = volume of gas = 22.4 L
T = temperature of gas =
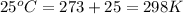
R = gas constant = 0.0821 Latm/moleK

similarly, we have to calculate the pressure of water vapor.

where,
 = mass of water vapor = 36 g
= mass of water vapor = 36 g
 = molar mass of water = 18 g/mole
= molar mass of water = 18 g/mole

Now we have to calculate the total pressure of gas.

 (1 atm = 760 torr)
(1 atm = 760 torr)
Therefore, the pressure of gas will be, 2904.72 torr