Answer: gradual increase in temperature of ice from −15°C to 0°C, holding at 0°C as ice melts to water
Explanation: The heat used to convert solid to liquid is gained by molecules to gain kinetic energy and thus the molecules start moving faster and the temperature increases.
But as the melting point is approached i.e.
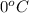 , the temperature remains constant as now the heat given is used to break the inter molecular forces and thus is not reflected in terms of temperature increase. This hidden heat is called as latent heat.
, the temperature remains constant as now the heat given is used to break the inter molecular forces and thus is not reflected in terms of temperature increase. This hidden heat is called as latent heat.
The temperature remains constant till the phase change is complete.