Answer:
changes in thermal energy
Step-by-step explanation:
A calorimeter is usually filled with water. Then, a hot material is placed in the water: the material transfer heat to the water, which is initially colder, until they have the same temperature (thermal equilibrium). By measuring the change in temperature of the water inside the calorimeter, it is possible to calculate the change in thermal energy (which is equal to the heat released by the material). In fact, the heat absorbed by the water is equal to the heat released by the material:

where
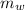 is the mass of the water
is the mass of the water
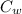 is the specific heat of the water
is the specific heat of the water
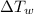 is the temperature change of the water
is the temperature change of the water
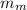 is the mass of the material
is the mass of the material
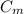 is the specific heat of the material
is the specific heat of the material
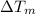 is the temperature change of the material
is the temperature change of the material
By knowing the mass of the water, its specific heat and by measuring the temperature change of the water, one can calculate the change in thermal energy.