Answer : The correct options are,
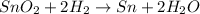 and
and
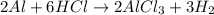
Explanation :
Single displacement reaction : It is a type of chemical reaction in which the more reactive element displaces the less reactive element.
Option A reaction :
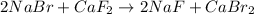
It is an example of double displacement reaction because in this reaction a positive cation and a negative anion of the two reactants exchange their places to form two new products.
Option B reaction :
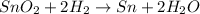
It is an example of single displacement reaction.
Option C reaction :
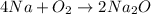
It is an example of combination reaction because in this reaction two reactants react to give a single product.
Option D reaction :
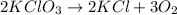
It is an example of decomposition reaction because in this reaction a single reactant decomposes into two or more products.
Option E reaction :
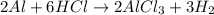
It is an example of single displacement reaction because in this reaction the most react element, aluminium displaces the less reactive element, hydrogen.
Hence, the options B and E are single displacement reactions.