1.70 × 10³ seconds
Explanation
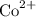 + 2 e⁻ →
+ 2 e⁻ →
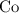
It takes two moles of electrons to reduce one mole of cobalt (II) ions and deposit one mole of cobalt.
Cobalt has an atomic mass of 58.933 g/mol. 0.500 grams of Co contains
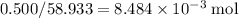 of Co atoms. It would take
of Co atoms. It would take
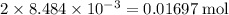 of electrons to reduce cobalt (II) ions and produce the
of electrons to reduce cobalt (II) ions and produce the
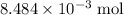 of cobalt atoms.
of cobalt atoms.
Refer to the Faraday's constant, each mole of electrons has a charge of around 96 485 columbs. The 0.01697 mol of electrons will have a charge of
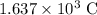 . A current of 0.961 A delivers 0.961 C of charge in one single second. It will take
. A current of 0.961 A delivers 0.961 C of charge in one single second. It will take
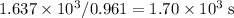 to transfer all these charge and deposit 0.500 g of Co.
to transfer all these charge and deposit 0.500 g of Co.