Answer :
(1) The mass of silver nitrate is, 555 g
(2) The solubility of the gas will be, 0.433 g/L
Solution for Part 1 :
From the given data we conclude that
In 100 gram of water, the amount of silver nitrate = 222 g
In 250 gram of water, the amount of silver nitrate =
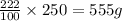
Therefore, the mass of silver nitrate is, 555 g
Solution for Part 2 :
Formula used :
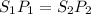 (at constant temperature)
(at constant temperature)
where,
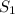 = initial solubility of methane gas = 0.026 g/L
= initial solubility of methane gas = 0.026 g/L
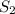 = final solubility of methane gas
= final solubility of methane gas
 = initial pressure of methane gas = 1 atm
= initial pressure of methane gas = 1 atm
 = final pressure of methane gas = 0.06 atm
= final pressure of methane gas = 0.06 atm
Now put all the given values in the above formula, we get the solubility of methane gas.
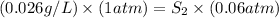
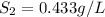
Therefore, the solubility of the gas will be, 0.433 g/L