8)
a.
p = 77
e = 77
n = 114
b.
Ir-191 has 114 neutrons, whereas, Ir-193 has 116 neutrons.
c.
Let the Percentage of Ir-191 in a sample be 'x', then, the Percentage of Ir-193 is (100 - x).
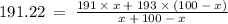
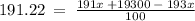
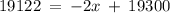
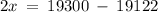
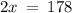
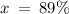
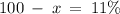
Therefore, in a naturally occurring Iridium sample, the amount of Ir-191 present is 89%, and the amount of Ir-193 present is 11%.
9)
False, Ir-191 is a common isotope having more no. of neutrons (114) than protons (77).