Answer: The magnitude of the electrostatic force between the ions is
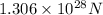 .
.
Step-by-step explanation:
Charge on
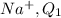 =+1 C
=+1 C
(sodium ion a has 11 protons and 10 electrons, 1 extra proton with positive charge )
Charge on
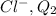 =-1 C
=-1 C
(Chlorine ion a has 17 protons and 18 electrons, 1 extra electron with negative charge)
Distance between the ions,r = 0.83 nm =
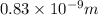
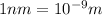
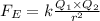
k=
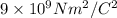
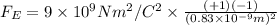
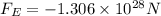
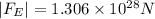
The magnitude of the electrostatic force between the ions is
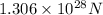 .
.