The average speed is calculated using the following formula
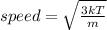
Where
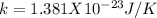
T = 298 K [room temperature]
m = mass of each molecule of oxygen
mass of one mole of oxygen molecules = 32 g / mol
mass of one molecule of oxygen will be =
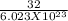
mass of one molecule of oxygen will be =
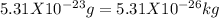
putting values
average speed =
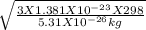
Average speed = 482.19 m / s
average speed = 1735.8 km / h
so approx = 1700 km/h