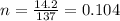1. Moles of 19.6 gram Aluminium
as we know that
Atomic mass of Al = 27 g/mol
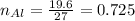
2. moles of 15.7 g Nitrogen
as we know that
Atomic mass of nitrogen = 28 g/mol
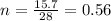
3. moles of 15.9 g Water
as we know that
Atomic mass of water = 2 + 16 = 18 g/mol
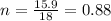
4. moles of 10 g Ammonia
as we know that
Atomic mass of Ammonia = 14+ 3 = 17 g/mol
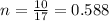
5. moles of 14.2 g Ammonium Phosphate
as we know that
Atomic mass of Ammonium Phosphate = 14(3) + 31 + 16(4) = 137 g/mol