Answer: D. 176 grams
Explanation:
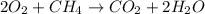
As methane is a limiting reagent as it limits the formation of product and oxygen is the excess reagent as it is in excess.
According to the given balanced equation,
1 mole of methane produces 1 mole of

Thus 4 moles of methane will produce 4 moles of

Mass of
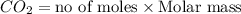
Mass of
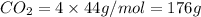
Thus 176 g of
 is produced in the reaction of 4 moles of
is produced in the reaction of 4 moles of
 in excess
in excess
 .
.