
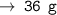
____________________________________
The given compound has 3 carbon atoms, so in 1 mole of that compound, there will be 3 moles of carbon atoms.
Mass of each mole of carbon atoms = 12 g
For 3 mole carbon atoms, it will be 12 × 3 = 36 grams
Answered by : ❝ AǫᴜᴀWɪᴢ ❞