Heat required = 173 kJ
Further explanation
Given
56.0 g of ice
Temperatur at 263 K(-10 C) to 400 K(127 C)
Required
Heat needed
Solution
1. raise the temperature(-10 C to 0 C)⇒c ice=2.09 J/g C
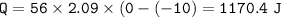
2. phase change (ice to water)⇒Heat of fusion water=334 J/g
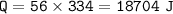
3. raise the temperature(0 C to 100 C)⇒c water= 4.18 J/g C
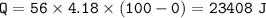
4. phase change(water to vapor)⇒heat of vaporization water=2260 J/g
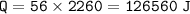
5. raise the temperature(100 C to 127 C)⇒c vapor=2.09 J/g C
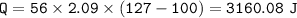
Total heat :
1170.4+18704+23408+126560+3160.08=173,002.48 J=173 kJ