Step-by-step explanation:
In quantum tunneling through a brier, the energy of the tunneled particle is same but the probability amplitude is decreased.
The expression for the probability of tunneling is expressed as follows:
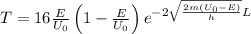
Here, T is the transmission probability; E is the kinetic energy, U_{0} is the barrier height, $m$ is the mass of the electron, and L is the width of the barrier.