Answer:
zince chloride will be formed and hydrogen gas will be librated.
Step-by-step explanation:
When dilute HCl is added to zinc pieces, a rection will takes place as follows :
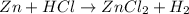
It means that zinc chloride will form when zinc reacts with dilute HCL. Also hydrogen gas will produced.
As zinc is more reactive than hydrogen, it displaces hydrogen from its solution and forms zinc chloride. The form product is white in color and H₂ is an odorless gas.
Hence, zince chloride will be formed and hydrogen gas will be librated.