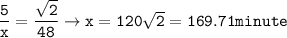169.71 minutes
Further explanation
Given
Rate of diffused of Hydrogen=5 gm/30 min
Required
The time required for SO₂
Solution
Graham's law: the rate of effusion of a gas is inversely proportional to the square root of its molar masses or
the effusion rates of two gases = the square root of the inverse of their molar masses:
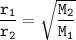
r₁=5gm/30 min
M₁=molar weight of H₂-hydrogen= 2 g/mol
M₂=molar weight of SO₂-sulfur dioxide= 64 g/mol
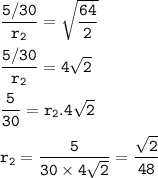
the time required (for the same amount=5 gm) :