Answer:
= 64584.7176 g
or
= 64.5847 kg
Step-by-step explanation:
From our question, we have been asked to find the mass of SO₂ containing 6.075 × 10^26 S atoms.
We know that from Avogadro's constant,
1 mole of a substance contains 6.02 x 10²³ ions/atoms/units
Thus,
1 mole => 6.02 x 10²³ atoms
x moles=> 6.075 × 10²⁶ atoms
We cross multiply;
x moles = (6.075 × 10²⁶ x 1)/(6.02 x 10²³)
= 1009.1362 moles
1 mole of SO2 = 64 g
1009.1362 moles = ?
=>
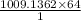
= 64584.7176 g
= 64.5847 kg