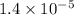The given question is incomplete. The complete question is:
The pH of a 0.98 M solution of propanoic acid is measured to be 2.43. Calculate the acid dissociation constant of propanoic acid. Round your answer to two significant digits.
Answer: The acid dissociation constant of propanoic acid is
Step-by-step explanation:
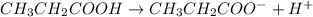
cM 0 0

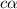
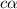
So dissociation constant will be:
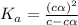
Give c= 0.98 M and pH = 2.43
![pH=-log[H^+]](https://img.qammunity.org/2021/formulas/chemistry/college/wyj0nahkywle04sx44478osqilvygxax2t.png)
![2.43=-log[c* \alpha]](https://img.qammunity.org/2021/formulas/chemistry/college/1nyy92h24vpitr87f4a33k6e8chg172a86.png)
![2.43=-log[0.98* \alpha]](https://img.qammunity.org/2021/formulas/chemistry/college/rwbi8twj498qulj2zm2rmowqzuuiduxkk5.png)
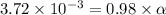
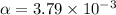
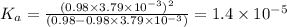
The acid dissociation constant of propanoic acid is