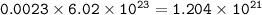The number of gold atom : 1.204 x 10²¹
Further explanation
Given
0.6 g of 18 carat gold
mass number of gold = 197
24 carat as 100% gold
Required
The number of gold atom
Solution
Percentage of gold in 18 carat :
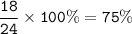
Mass of gold in 0.6 g of 18 carat :
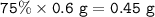
mol :
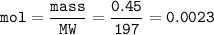
A mole is a number of particles(atoms, molecules, ions) in a substance
1 mole = 6.02.10²³ particles
So the number of gold atom :