Answer:
n = 0.219 moles
Step-by-step explanation:
Given mass, m = 32.5 g
Molar mass of Mg(NO₃)₂ is :
M = 1 × 24 + 2× 14 + 6 × 16
M = 148 g/mol
Let there are n number of moles. It is equal to given mass divided by molar mass.
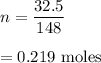
Hence, there are 0.219 moles in 32.5 g of Mg(NO₃)₂.