Answer:
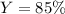
Step-by-step explanation:
Hello!
In this case, since we know the balanced chemical reaction, we are first able to realize there is a 1:3 mole ratio between zinc phosphate and zinc chloride; it means that we can first compute the moles of the desired product via stoichiometry:
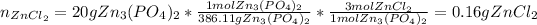
Next, since those moles are associated with the theoretical yield of zinc chloride, we obtain the corresponding mass:
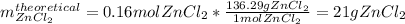
Finally, we compute the percent yield by diving the actual yield (18 g) by the theoretical yield:
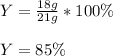
Best regards!