Answer:
3.375moles of O₂
Step-by-step explanation:
The reaction expression is given as;
2KClO₃ → 2KCl + 3O₂
Number of moles of KClO₃ = 2.25moles
Now;
To find the number of moles of O₂ produced we use known number of moles.
So;
2 mole of KClO₃ will produce 3 moles of O₂
2.25moles of KClO₃ will produce
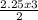 = 3.375moles of O₂
= 3.375moles of O₂