Given :
A copper plate of mass 3.0g.
Atomic number, z = 29.
Atomic mass, M.M = 63.5 g/mol.
Avogadro's number,
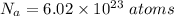 .
.
Charge on electron,
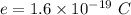 .
.
To Find :
The total charge of all the electrons.
Solution :
Moles of copper,
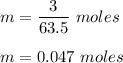
Number of atoms in 0.047 moles of copper :
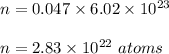
Since, there are 29 electrons on each atoms.
So, total charge is :
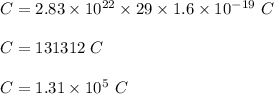
Hence, this is the required solution.