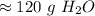Answer:

Step-by-step explanation:
We want to convert from moles of water to grams of water.
First, find the molar mass of water (H₂O) Look on the Periodic Table for the masses of hydrogen and oxygen.
- Hydrogen (H): 1.008 g/mol
- Oxygen (O): 15.999 g/mol
Next, add up the number of each element in water. The subscript of 2 comes after the H, so there are 2 moles of hydrogen.
- 2 Hydrogen: (1.008 g/mol*2) = 2.016 g/mol
Finally, add the molar mass of 2 hydrogen and 1 oxygen.
- 2.016 g/mol (2 Hydrogen) + 15.999 g/mol (1 oxygen)= 18.015 g/mol
Next, find the grams in 6.5 moles.
Use the molar mass we just found as a ratio.
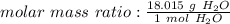
We want to find the grams in 6.5 moles. We can multiply the ratio above by 6.5
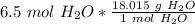
Multiply. Note that the moles of H₂O will cancel each other out.
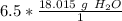
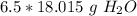
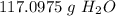
If we want to round to the technically correct significant figures, it would be 2 sig figs. The original measurement, 6.5, has 2 (6 and 5).