Answer:
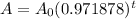
Explanation:
The half-life of Phosphorus-32 is approximately 24.3 days.
We have the equation:
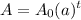
And we would like to determine a.
First, since it is half-life, a=1/2.
Second, since the half-life for our element is 24.3 days, we need to substitute t for t/24.3. This yields:
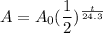
In this way, if t=24.3, then we would have one half-life.
We can rewrite our equation as:
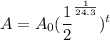
Approximate, rouding to six decimal places. So, our function is:
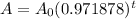
Therefore, our new a is:
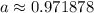
This will give the amount of Phosphorus-32 left after t days, giving the initial amount of A₀.