Answer:
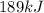
Step-by-step explanation:
Hello!
In this case, since one mole of ethanol release 8,842 J per 1 mole of ethanol, we can write:
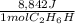
Thus, since we need the energy released by 982.6 g of ethanol, we compute the moles in such mass of fuel:
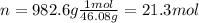
Therefore, the result is:
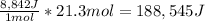
Which in kJ is:
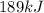
Best regards!