Answer: A. They are not reactive because their outer valence electron shell is full.
Step-by-step explanation:
Noble gases are group 18 elements which are Helium , Neon , Argon , Krypton , Xenon and radon.
Noble gases have general electronic configuration of
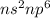
As the sub-shells of noble gases is fully filled and their octet is complete. Hence, it will not gain or lose any electron and thus is non reactive in nature.
They do not react with other elements under ordinary conditions.
Thus the correct statement is they are not reactive because their outer valence electron shell is full.