Answer:
7.8%
Step-by-step explanation:
Given that:
The initial mass amount of aspirin = 0.020g
The standard molar mass of aspirin = 240 g/mol
Thus, the number of moles = mass/molar mass
= 0.020/240
= 0.0000833 moles
Now, the molarity of aspirin in the solution(diluted)
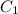 =
=
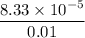
=
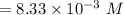 (provided the volume v = 0.01 L)
(provided the volume v = 0.01 L)
The absorbance of the sample solution A =1.07
The path length (b) = 1 cm
From the standard value of salicylic acid, the coefficient (e)= 139.322 /M/cm
Now; according to Beer's law, the concentration of aspirin is:
A = e×b×c
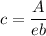
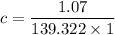
c = 0.00768 M
Finally, relating the concentration of the aspirin, the percentage of salicylic acid the product
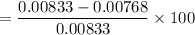
= 7.8%