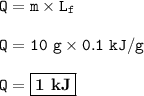Heat needed to to melt 10 g of ethanol : 1 kJ
Further explanation
The heat to change the phase can be formulated :
Q = mLf (melting/freezing)
Q = mLv (vaporization/condensation)
Lf=latent heat of fusion
Lv=latent heat of vaporization
mass of ethanol= 10 g
Heat needed to melt :