Answer:
588.2 mL
Step-by-step explanation:
- FeSO₄(aq) + 2KOH(aq) → Fe(OH)₂(s) + K₂SO₄(aq)
First we calculate how many Fe⁺² moles reacted, using the given concentration and volume of FeSO₄ solution (the number of FeSO₄ moles is equal to the number of Fe⁺² moles):
- moles = molarity * volume
- 187 mL * 0.692 M = 129.404 mmol Fe⁺²
Then we convert Fe⁺² moles to KOH moles, using the stoichiometric ratios:
- 129.404 mmol Fe⁺² *
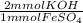 = 258.808 mmol KOH
= 258.808 mmol KOH
Finally we calculate the required volume of KOH solution, using the given concentration and the calculated moles:
- volume = moles / molarity
- 258.808 mmol KOH / 0.440 M = 588.2 mL