Further explanation
1.Atomic Number (Z) = Mass Number (A) - Number of Neutrons
neutrons = mass number-atomic number
Atomic mass Cl-37= 17
Mass number Cl-37=37
Neutrons = 37-17=20
2. Mass atom X = mass isotope 1 . % + mass isotope 2.%...
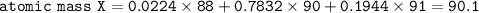
3. The energy in one photon can be formulated as
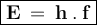
f = c / λ, so :
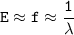
Energy is directly proportional to frequency and inversely proportional to the wavelength
So, as the frequency of photon increases, the energy of photon increases
4. Based on answer number 3 :
A. The wavelength becomes longer, and the energy decreases