Answer:
Step-by-step explanation:
The given compound is:
KC₈H₆O₄
Mass of the compound = 25g
Molar mass of the compound
KC₈H₆O₄
Atomic mass of K = 39
C = 12
H = 1
O = 16
Molar mass of the compound = 39 + 8(12) + 6 (1) + 4(16)
= 205g/mol
Number of molecules of fuel there in:
Find the number of moles then convert this to number of molecules;
Number of moles =

Number of moles =
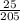 = 0.12mol
= 0.12mol
1 mole of a compound contains 6.02 x 10²³ molecules
0.12 mole of the phthalate will contain 0.12 x 6.02 x 10²³ =
7.34 x 10²²molecules
Number of moles of Carbon
1 mole of the phthalate contains 8 mole of carbon
0.12 mole of the phthalate will contain 0.12 x 8 = 0.96mole of carbon