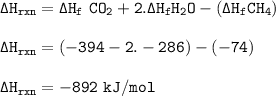The enthalpy change of combustion of methane : -892 kJ/mol
Further explanation
The change in enthalpy in the formation of 1 mole of the elements is called enthalpy of formation
The enthalpy of formation measured in standard conditions (25 ° C, 1 atm) is called the standard enthalpy of formation (ΔHf °)
Determination of the enthalpy of formation of a reaction can be through a calorimetric experiment, based on the principle of Hess's Law, enthalpy of formation table, or from bond energy data
The value of ° H ° can be calculated from the change in enthalpy of standard formation:
∆H ° rxn = ∑n ∆Hf ° (product) - ∑n ∆Hf ° (reactants)
Reaction for combustion of Methane (CH₄) :
CH₄ + 2O₂ → CO₂ + 2H₂O.
The elements in standard conditions are not included in the enthalpy calculations because the enthalpy of those elements under the standard conditions is zero(∆Hf °O₂ =0)
∆Hf ° CO₂ = -394 kj/mol
∆Hf ° H₂O=-286 kj/mol
∆Hf ° CH₄=-74 kJ/mol
The enthalpy change of combustion of methane :