Answer: A. 5.41
Step-by-step explanation:
To calculate the moles :
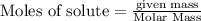
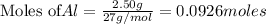
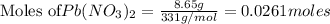
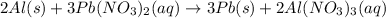
According to stoichiometry :
3 moles of
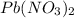 require = 2 moles of
require = 2 moles of
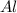
Thus 0.0261 moles of
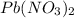 will require=
will require=
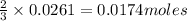 of
of
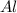
Thus
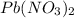 is the limiting reagent as it limits the formation of product and
is the limiting reagent as it limits the formation of product and
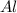 is the excess reagent.
is the excess reagent.
As 3 moles of
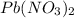 give = 3 moles of
give = 3 moles of
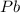
Thus 0.0261 moles of
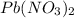 give =
give =
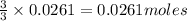 of
of
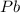
Mass of
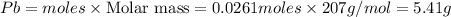
Thus 5.41 g of solid lead will be produced from the given masses of both reactants.