Answer:
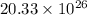 atoms
atoms
Step-by-step explanation:
Given that,
Given mass = 13200 g
Molar mass of potassium = 39.0983 g/mol
Let there are n number of moles. It is equal to mass divided by molar mass. It is equal to :
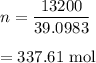
Let there are x atoms in 13200 g of potassium (k). It can be calculated :
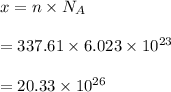
So, there are
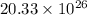 atoms.
atoms.