Answer: Decomposition reaction.
Step-by-step explanation:
Decomposition is a type of chemical reaction in which one reactant forms two or more than two products.
Decomposition reactions require breaking of bonds which require energy and thus all of the decomposition reactions are endothermic reactions. They require energy in the form of heat, electricity or sunlight and thus called as thermal, electrolytic and photolytic decomposition respectively.
The given reaction
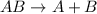 is a type of decpomposition reaction.
is a type of decpomposition reaction.