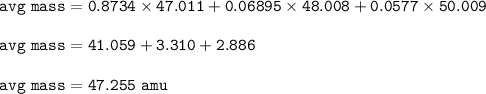The average atomic mass of the imaginary element : 47.255 amu
Further explanation
The elements in nature have several types of isotopes
Isotopes are elements that have the same Atomic Number (Proton)
Atomic mass is the average atomic mass of all its isotopes
Mass atom X = mass isotope 1 . % + mass isotope 2.% ..
isotope E-47 47.011 amu, 87.34%
isotope E-48 48.008 amu, 6.895
isotope E-49 50.009 amu, 5.77%
The average atomic mass :