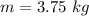Answer:
The mass is
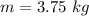
Step-by-step explanation:
From the question we are told that
The initial temperature is
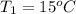
The final temperature is
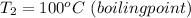
Generally the maximum heat produced by 1 Liter of natural gas is
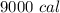
So the amount of heat produced by 100 L is
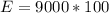
=>
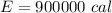
Generally given that the efficiency is
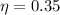
Then actual heat received by the water is
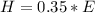
=>
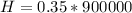
=>
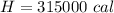
Converting to kcal
=>
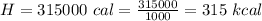
Generally the specific heat of water is
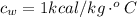
Generally the heat received by the water is mathematically represented as
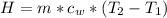
=>
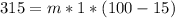
=>