Question 1
1) The formula of iron(II) iodide is
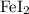 and has a formula mass of 309.654 g/mol. This means that in 12.2 grams, there are
and has a formula mass of 309.654 g/mol. This means that in 12.2 grams, there are
- 12.2/309.654 = 0.039398812868557 mol.
So, the molarity is (0.039398812868557)/(0.5) = 0.0788 M (to 3 sf)
2) In 0.039398812868557 mol of iron(II) iodide, there are 0.039398812868557 moles of iron(II) iodide cations, and thus the molarity is still 0.0788 M (to 3 sf)
3) In 0.039398812868557 mol of iron(II) iodide, there are
- 2(0.039398812868557) = 0.078797625737114 moles of iodide anions
Thus, the molarity is (0.078797625737114)/(0.5) = 0.158 M (to 3 sf)
Question 2
1) The formula of iron(III) sulfate is
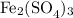 and has a formula mass of 399.9 g/mol. This means that in 16.8 grams, there are
and has a formula mass of 399.9 g/mol. This means that in 16.8 grams, there are
- 16.8/399.9 = 0.042010502625656 mol
So, the molarity is (0.042010502625656)/(0.25) = 0.168 M (to 3 sf)
2) In 0.042010502625656 moles of iron(III) sulfate, there are 2(0.042010502625656)=0.084021005251312 moles of iron(III) cations, so the molarity is:
- 0.084021005251312/0.25 = 0.336 M (to 3 sf)
3) In 0.042010502625656 moles of iron(III) sulfate, there are 3(0.042010502625656)=0.12603150787697 moles of sulfate anions, so the molarity is:
- 0.12603150787697/0.25 = 0.504 M (to 3 sf)
Question 3
Since we need 50.0 mL of solution at 0.271 M,
- 0.271 = (moles of HBr)/(0.05)
- moles of HBr = 0.01355 mol
So, we need this from 12.0 M HBr so, meaning that:
- 12.0 = (0.01355)/(liters of 12.0 M HBr stock solution)
- liters of 12.0 M HBr stock solution = 0.0011291666666667 L = 1.13 M (to 3 sf)