Answer:
0.56L
Step-by-step explanation:
This question requires the Ideal Gas Law:
 where P is the pressure of the gas, V is the volume of the gas, n is the number of moles of the gas, R is the Ideal Gas constant, and T is the Temperature of the gas.
where P is the pressure of the gas, V is the volume of the gas, n is the number of moles of the gas, R is the Ideal Gas constant, and T is the Temperature of the gas.
Since all of the answer choices are given in units of Liters, it will be convenient to use a value for R that contains "Liters" in its units:
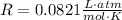
Since the conditions are stated to be STP, we must remember that STP is Standard Temperature Pressure, which means
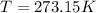 and
and
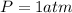
Lastly, we must calculate the number of moles of
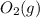 there are. Given 0.80g of
there are. Given 0.80g of
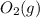 , we will need to convert with the molar mass of
, we will need to convert with the molar mass of
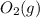 . Noting that there are 2 oxygen atoms, we find the atomic mass of O from the periodic table (16g/mol) and multiply by 2:
. Noting that there are 2 oxygen atoms, we find the atomic mass of O from the periodic table (16g/mol) and multiply by 2:
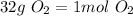
Thus,
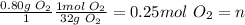
Isolating V in the Ideal Gas Law:

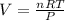
...substituting the known values, and simplifying...
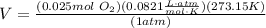
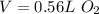
So, 0.80g of
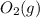 would occupy 0.56L at STP.
would occupy 0.56L at STP.