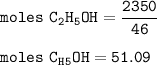There are 51.09 moles of C₂H₅OH in the fuel tank
Further explanation
A mole is a number of particles(atoms, molecules, ions) in a substance
This refers to the atomic total of the 12 gr C-12 which is equal to 6.02.10²³, so 1 mole = 6.02.10²³ particles
Can be formulated :
N = n x No
N = number of particles
n = mol
No = 6.02.10²³ = Avogadro's number
moles can also be obtained by dividing the mass (in grams) with the molar mass
m = mass
M = relative molecular mass / molar mass
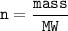
The fuel tank contains 2350 g of C₂H₅OH, so mol C₂H₅OH(MW= 46 g/mol) :