Heat needed : 159.33 kJ
Further explanation
The heat to change the phase can be formulated :
Q = mLf (melting/freezing)
Q = mLv (vaporization/condensation)
Lf=latent heat of fusion
Lv=latent heat of vaporization
The heat needed to raise the temperature
Q = m . c . Δt
The specific heat of ice is 2.09J/g°C; the specific heat of water is 4.182 J/g°C; the heat of fusion is 333.0 J/g.
1. heat to raise temperature from -35 °C to 0 °C

2. phase change(ice to water)
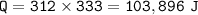
3. heat to raise temperature from 0 °C to 25 °C
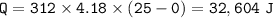
Total heat needed :
22,828.8 + 103,896+32,604=159,328.8 J=159.33 kJ