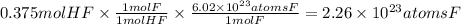Answer:
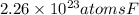
Step-by-step explanation:
Step 1: Given data
- Molarity of the HF solution (M): 0.500 M
- Volume of the solution (V): 750.0 mL
Step 2: Convert "V" to liters
We will use the conversion factor 1 L = 1000 mL.
750.0 mL × 1 L/1000 mL = 0.7500 L
Step 3: Calculate the moles of HF
We will use the following expression.
n = M × V
n = 0.500 mol/L × 0.7500 L = 0.375 mol
Step 4: Calculate the atoms of F in 0.375 moles of HF
We will use the following relationships:
- 1 mole of HF contains 1 mole of atoms of F.
- 1 mole of atoms of F contains 6.02 × 10²³ atoms of F (Avogadro's number).