The atomic mass of iron is 55.845 g/mol, so 10.0 g is equal to
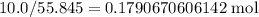 .
.
From the equation, we know that for every 2 moles of iron consumed, 3 moles of chlorine are consumed.
This means we need
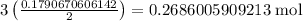 chlorine.
chlorine.
Chlorine has an atomic mass of 35.45 g/mol, so
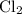 has a formula mass of 35.45(2)=70.9 g/mol.
has a formula mass of 35.45(2)=70.9 g/mol.
Thus, the mass of chlorine needed is (0.2686005909213)(70.9), which is about 19.0 g (to 3 sf)