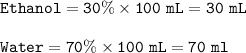Volumes of ethanol and water are in the solution : 30 mL and 70 ml
Further explanation
The concentration of a substance can be expressed in several quantities such as moles, percent (%) weight/volume,), molarity, molality, parts per million (ppm) or mole fraction. The concentration shows the amount of solute in a unit of the amount of solvent.
Percent Volume (% v/v) : volume (ml) of solute/100 ml of solution ⇒ ratio of volume of the solute to total volume of the solution
 .
.
30% ethanol ⇒30% etanol, 70% water
In 100 mL solution :