The hydroxide ion concentration, [OH-] = 2.439 x 10⁻¹⁰
Further explanation
Equilibrium of water and the ions :
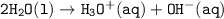
![\tt Kc=([H_3O^+][OH^-])/(H_2O)](https://img.qammunity.org/2021/formulas/chemistry/college/fcytih2dl5lbfnaiu8plm0j1cl8b8kk1i7.png)
Kc[H₂O] is the ion equilibrium constant for water ⇒ Kw.
The product of the concentrations of these ions (H₃O⁺ and OH⁻)at equilibrium at 25°C is 1.0 x 10⁻¹⁴ M
[H₃O⁺] [OH⁻] = 1.0 x 10⁻¹⁴ M = Kw
The hydrogen ion concentration of [H⁺]=4.1 x 10⁻⁵ M, so the hydroxide ion concentration, [OH-] :
![\tt 4.1* 10^(-5)* [OH^-]=1.10^(-14)\\\\(OH^-]=(1.10^(-14))/(4.1* 10^(-5))\\\\(OH^-]=2.439* 10^(-10)](https://img.qammunity.org/2021/formulas/chemistry/college/d1bk37i18i633g7dltg0s8rqwwnd6jv0c7.png)