[H₃O⁺] = 8.86 x 10³ M
Further explanation
The dissociation of water :
H₂O(l)⇒H⁺+OH⁻
Hydrogen ions H⁺ bond with H₂O to form H₃O⁺ ions(hydronium ion)
When acid(Hbr acid) is in the presence of water(dissociates), the H⁺ ions bond with water molecules to form hydronium, the reaction :
HBr(aq)+H₂O→H₃O+(aq)+Br−(aq
So Ionization of HBr :
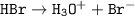
[HBr]= 8.86 x 10³,
From equation ratio [H₃O⁺] : HBr = 1 : 1, so
![\tt [H_3O^+]=8.86* 10^3](https://img.qammunity.org/2021/formulas/chemistry/college/qecpmuebhqnn1csetztdk17ddskw7im7bu.png)