There are 1825.6 g in a 14.5 moles Lithium permanganate
Further explanation
The mole is the number of particles(molecules, atoms, ions) contained in a substance
1 mol = 6.02.10²³ particles
Can be formulated
N=n x No
N = number of particles
n = mol
No = Avogadro's = 6.02.10²³
Moles can also be determined from the amount of substance mass and its molar mass :
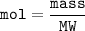
moles of Lithium permanganate = 14.5
Lithium permanganate (LiMnO4) MW=125.9 g/mol, so mass :
