441 g CaCO₃ would have to be decomposed to produce 247 g of CaO
Further explanation
Reaction
Decomposition of CaCO₃
CaCO₃ ⇒ CaO + CO₂
mass CaO = 247 g
mol of CaO(MW=56 g/mol) :
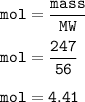
From equation, mol ratio CaCO₃ : CaO = 1 : 1, so mol CaO :
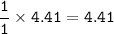
mass CaCO₃(MW=100 g/mol) :
